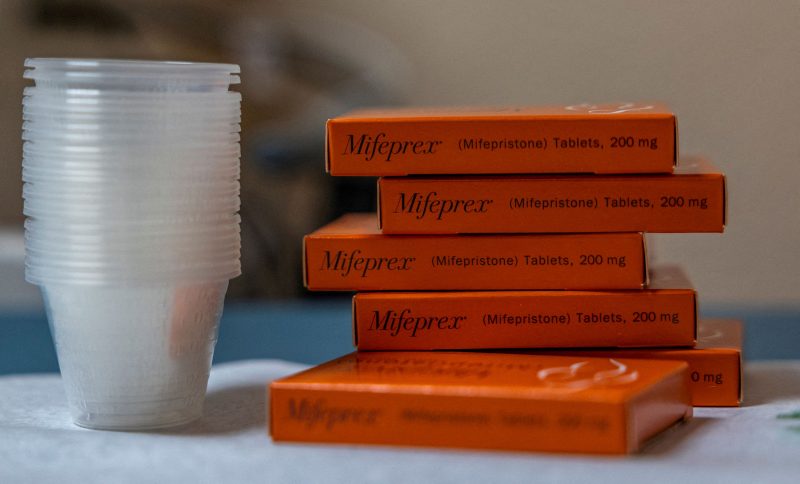
Dozens of Republican and Democratic attorneys general filed competing arguments on Friday in a heated Texas lawsuit that seeks to reverse the Food and Drug Administration’s approval of abortion pills, the most common method to terminate pregnancy in the United States.
Mississippi Attorney General Lynn Fitch (R), with 21 attorneys general from red states, argued in a brief that the FDA “undermined the public interest” by allowing the medication. New York Attorney General Letitia James (D), also with 21 co-signers, said in court filings that revoking the drug’s approval would have “devastating consequences” for Americans. The clashing briefs are the latest in rising tensions around the case, which could undo the FDA’s decades-old approval of mifepristone, the drug used in medication abortions.
The lawsuit was filed in November by conservative group Alliance Defending Freedom in the U.S. District Court for the Northern District of Texas. It argues that the FDA lacked the authority to approve mifepristone and did not thoroughly study the medication, while claiming that the drug is unsafe.
U.S. District Court Judge Matthew Kacsmaryk is presiding over the case. He was nominated by President Donald Trump and is known for his conservative views on issues like same-sex marriage and abortion. He could rule as early as next week. An appeal would go to the right-leaning Fifth Circuit Court of Appeals, and could eventually present the Supreme Court with another major abortion case less than a year after it upended Roe v. Wade.
Arguing for the Democratic attorneys general, James in a statement said that “decades of medical and clinical research have proven that medication abortion is safe.”
“Blocking access to this safe and effective medication is a dangerous attack on reproductive freedom and public health,” James said. “Access to safe reproductive health care could be in jeopardy for millions of Americans because of a baseless lawsuit.”
On the Republican side, Fitch stated that the FDA is defying federal and state laws in its decision to allow access to the drug.
“In the Dobbs case, the Supreme Court affirmed that states may enact laws that protect unborn life, women’s health and the integrity of the medical profession, and we will not allow the Biden administration to trample on this fundamental constitutional building block,” Fitch said.
The Biden administration and abortion rights advocates denounced the suit, and the FDA has since eased access to medication abortion in states where it is legal by allowing retail pharmacies to dispense the pills.
The Republican attorneys general brief criticizes these efforts, stating: “Rather than respect the Constitution, the Supreme Court, and the democratic process, the Biden Administration has attacked and worked to undermine” states’ elected representatives.
Meanwhile, the Democratic attorneys general brief argues that annulling the FDA’s approval of mifepristone would “eviscerate” states’ decisions to protect abortion access.
Medication abortion has come under increasing fire since the Supreme Court overturned Roe in June.
The FDA approved the medication in 2000 as safe and effective through the first seven weeks of pregnancy. In 2016, that was extended to 10 weeks. Mifepristone blocks the hormone progesterone, which is needed to sustain a pregnancy. Patients follow the use of mifepristone with misoprostol, which causes the uterus to empty.
The Texas suit challenges the FDA’s relaxing of restrictions on the abortion pill, contending that the agency chose “politics over science” in its decades-old decisions.